
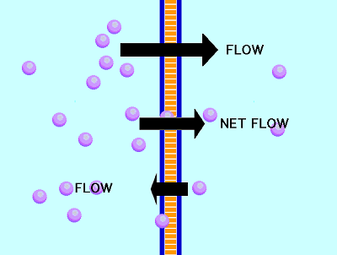
Living things can be shown to obey the second law if a little complexity is taken into account, however. It is also difficult to reconcile conceptually with the phenomenon of life, in which complex molecules and structures seem to assemble themselves from simpler components in contradiction to the second law. This is not a comforting thought but it is intuitive to most of us. The second law of thermodynamics states that for any physical or chemical process, the entropy (S, "disorder") of the universe increases. In this case, some of the light energy is converted to heat and some is converted back into light (fluorescence). from light to chemical bond energy, the conversion is rarely 100% efficient. There is never a need to account for energy that disappears from the universe, for example.Ī point to make at this juncture is that when energy changes forms, e.g. This is a useful fact when you are calculating energy flows. The first law states that energy is never created or destroyed in any physical or chemical process but it may change forms. A basic knowledge of them is required to understand bioenergetics. Several laws of thermodynamics have been developed. They are related, allowing us to see that biological chemistry is the same as any other chemistry, just more complex. Entropy is the disorder of a system.įree energy can quantify the potential work that a system can Hence the continuous need for synthesis of ATP in livingĬoncepts from General Chemistry. Hydrolysis of ATP is necessary for many reactions to proceed, Monomers into starch must be accompanied by hydrolysis of ATP intoĪDP + inorganic phosphate. Reactions that create order must be coupled to other reactions that create more disorder.Absorption of a photon or oxidation of another molecule are required for these reduction reactions to proceed. Reduced forms of these molecules have more energy than their oxidized forms, therefore reducing them requires a second event that supplies the energy. There are many biological molecules that can gain electrons, becoming reduced, and lose electrons, becoming oxidized. Reduced molecules have more energy than their oxidized forms.Positively charged ions enter if a channel protein in the membrane is open, even against the concentration gradient. For example, all plant cells are negatively charged inside.
This can be counterintuitive, expecially if ions move against their concentration gradient to equalize the charge gradient.

Thermodynamics, in turn, is the study of energy flow through systems. Reading: Chapter 2, Taiz and Zeiger's Plant Physiologyīioenergetics is the name given to the study of thermodynamics in living things.


 0 kommentar(er)
0 kommentar(er)
